Abstract
Allogeneic stem cell transplantation (allo-HSCT) to treat hematological malignancies can induce life-threatening complications, such as graft-versus-host disease (GvHD). Steroids remain the first-line treatment for acute GvHD (aGvHD). Nevertheless, new treatments are needed to, not only, treat steroid-resistant patients and improve allo-HSCT outcomes, but also reduce steroid complications. Increasing evidences strongly suggest that gut microbiota composition and the activity of regulatory T cells (Tregs) could be involved in GvHD prevention.
We have identified a novel FoxP3-negative IL-10-secreting Treg subset, named DP8α, enriched in the colonic mucosa and present in blood. These cells display a TCR-specificity for the gut ClostridiumIV bacterium Faecalibacterium prausnitzii. Sizable fractions of these cells also expressed the membrane-bound ectonucleotidases CD39 and CD73, which are directly involved in their suppressive activity in vitro. In vivo, these enzymes play key regulatory roles by both degrading proinflammatory extracellular ATP and inducing the production of immunosuppressive adenosine. An alteration in this adenosine/purinergic pathway has also been linked to GvHD occurrence.
Altogether, these data prompted us to hypothesize that F. prausnitzii-reactive DP8α Tregs, whose suppressive activity is driven by the purinergic pathway, could bridge microbiota dysbiosis and GvHD incidence in allo-HSCT patients. Moreover, decreased levels of Clostridia, especially Faecalibacteriumspp, in patient's stools, have been associated with aGvHD risk, and mice gavaged with Treg-inducing Clostridia displayed milder GvHD and improved survival.
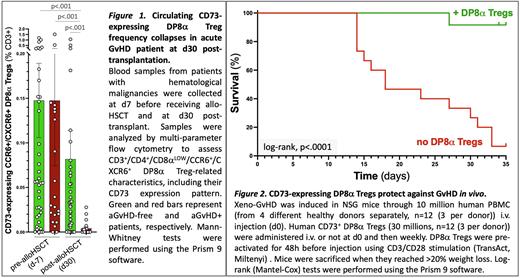
Therefore, we addressed a potential role for DP8α Tregs in the prevention of aGvHD. We first analyzed cells from 63 patients with hematological malignancies, who received allo-HSCT, among whom a third developed aGvHD. We quantified their circulating DP8α Tregs and their CD39 and CD73 expression pre- and post-allo-HSCT. While the percentage of CD73-expressing DP8α Tregs within CD3+ T cells before HSCT was not different, a strikingly deficiency of CD73-expressing DP8α Tregs was strongly associated with aGvHD development (p<.001) at 1-month post-transplant, as compared to aGvHD-free patients (Fig.1). Importantly, CD73 expression was not affected on any other T cell subset analyzed. CD73 decrease on DP8α Tregs did not result from corticotherapy since it was 1/ equally observed before and after treatment in aGvHD patients and 2/ not observed following in vitro treatment of PBMCs with methylprednisolone. Moreover, the decrease of CD73-expressing DP8α Tregs in aGvHD patients was observed across all treatment conditions, which were heterogeneous, and therefore appears relevant and likely related to the aGvHD condition itself. Altogether, these data strongly suggest that a CD73-dependent functional alteration of DP8α Tregs could, at least in part, be involved in aGvHD occurrence and/or development.
We next evaluated the therapeutic efficacy of CD73+ DP8α Tregs in a pre-clinical model of acute xeno-GvHD induced through human PBMC injection in irradiated NSG recipient mice. In vitro-expanded CD73+ DP8α Treg injected i.v. drastically protected mice against xeno-GvHD (Log-rank, p<.0001; Fig.2) without preventing the development of human chimerism. Moreover, these in vivo experiments, repeated 4 times using different PBMC donors, all confirmed the potent protective role of CD73+ DP8α Tegs in this model. Accordingly, serum inflammatory human cytokines and tissue infiltration by human cells were significantly lower in mice treated with CD73+ DP8α Tregs, as compared to untreated mice. In addition, the colon was rapidly populated with the injected DP8α Tregs and well-preserved in treated mice, while clearly damaged in untreated mice.
Altogether, these results strongly support a role for CD73+ DP8α Tregs in aGVHD prevention and advocate for the use of these cells to both predict aGvHD risks and give rise to the development of innovative therapeutic strategies to preclude GvHD-related inflammation. Such therapeutic approaches would be based on the infusion of DP8α Tregs and/or their in vivo stimulation, e.g., with F. prausnitzii-derived antigens or probiotics. Of note, DP8α Tregs display an uniquely high proliferating potential and stable immunosuppressive functions in vitro, a key feature to implement such therapeutics.
Disclosures
Chevallier:Pfizer: Research Funding; Jazz Pharmaceuticals: Honoraria; Abbvie: Honoraria; Takeda: Honoraria; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.